Hydrohesive® is a type of hydrogel patch manufactured
by Teikoku Seiyaku Group.
We are proud of the transdermal technology, quality,
and tradition that we have cultivated since our founding.
The word Hydrohesive® is a formed by combining the two English words “hydrophilic” and “adhesive”. It is a collective term for Teikoku Seiyaku’s hydrogel patches including ethical drugs, OTC drugs, cosmetics, and cooling sheets in the form of hydrogel patches.
We at Teikoku Seiyaku are confident and proud of the high quality that we have cultivated over our long history and will continue to lead the industry and contribute to the world as a leader in hydrogel patch formulation innovation.
Hydrohesive® through the years
- Transdermal technology expertise refined through tradition and history since 1848
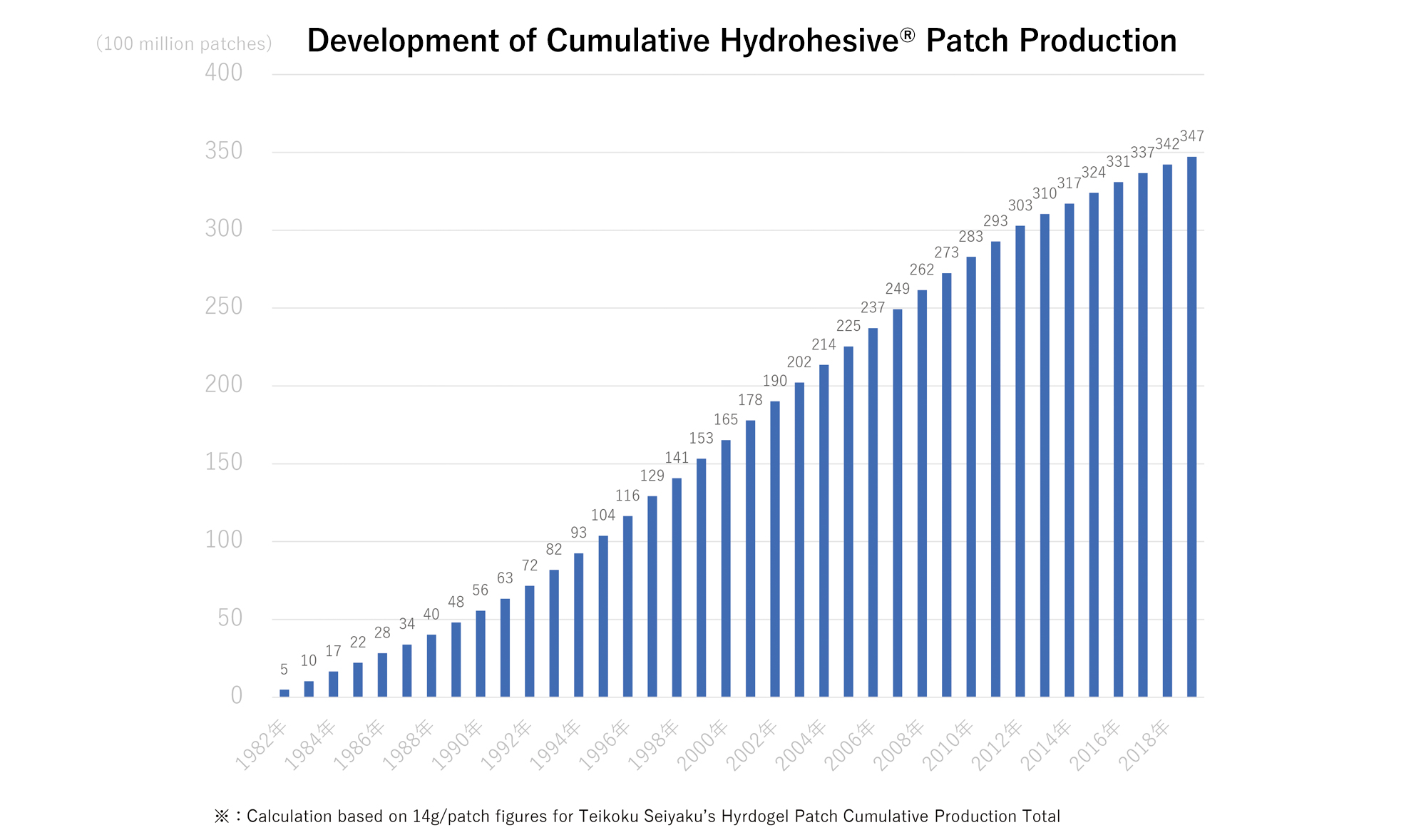
- In Teikoku Seiyaku’s history, we have manufactured over 34.7 billion*1 hydrogel patches using Hydrohesive® technology.
- Over 4 billion*2 Lidocaine patches using Hydrohesive® technology*2 including “Lidoderm®” for the US, its AG*3 equivalent, “Versatis®” for Europe, etc., have been marketed in more than 60 countries since 1999.
- In Japan, a cumulative total of more than 8 billion*4 Seltouch® Pap 70 products have been shipped since its launch in 1993.
History of Medicated Patches
Teikoku Seiyaku’s Hydrohesive® Technology
Teikoku Seiyaku is the world’s largest medicated patch manufacturer, having produced a cumulative total of over 34.7 billion patches*1 for both the Japanese and overseas markets. If you were to take the shorter sides of a typical patch (10cm x 14cm) and line up all the patches Teikoku Seiyaku has manufactured, it would be the equivalent distance of six return trips from Earth to the moon. Teikoku Seiyaku’s annual patch production capacity, using the same standard size of patch, is the equivalent length of around 4.2 times the circumference of the earth, at around 1.2 billion patches.
Teikoku Seiyaku’s medicated patches trace their origins back to the launch of the Horxis® product in 1938. During those days, clay wrapped around cloth formed poultices which were used to treat the patient’s affected area. There was a demand for an easier method of applying the poultice, so innovations were made so that each patch could be a standard size and the clay could be spread out and be absorbed by the cloth. This is how Panapap-L was created in 1974.

Launch of “Horxis®

“Panapap-L” added to NHI Drug Price Standard List
From this point forward, Teikoku Seiyaku set about further innovating and perfecting the manufacture of patches so that they would be easier for patients to use.
Through these innovations, Teikoku Seiyaku developed and received approval for the production of the world’s first Hydrohesive® lidocaine patch, Lidoderm® in 1999. From our manufacturing plant in Sanbonmatsu, Kagawa prefecture, Teikoku Seiyaku produced its lidocaine patch series for patients in over sixty countries, including Lidoderm® for the United States along with its Authorised Generic*4 equivalent and Versatis® in the European market with over 3 billion patches already produced. Teikoku Seiyaku’s record for production of patches for the American market still stands. Domestically, a cumulative total of 8 billion units of Seltouch® Patch 70, including under its former trade name, have been shipped since 1993.
- ※1:Based on cumulative production quantity when converted to 14g per sheet
- ※2:Based on cumulative shipment volume since 1999
- ※3:A cumulative total of Seltouch® Pap 70 and Seltouch® Pap 140 shipments, converted into the cumulative manufacturing volume of Seltouch® Pap 70 in terms of size (10cm x 14 cm)
- ※4:A generic drug identical to the brand-name drug, which has the same active ingredients, additives and manufacturing method, etc.